Quality Management System Design, Gap Analysis, Implementation, and Improvement
Gain Peace of Mind. Remove the Guesswork. Fill the Resource Gap.
Avoca’s Consulting Services for the design, development, and implementation of a Quality Management System (QMS) aims to help sponsors and CROs develop a culture of risk prevention rather than one of managing issues.
A proactive, risk-based approach ensures patient safety, improves data quality, provides data integrity, and increases inspection preparedness.
Why is a Quality Management System Important?
To be compliant with ICH E6 (R2) and (R3), risk-based quality management for clinical trials is required. A QMS is a framework for all activities, including quality control, quality assurance, quality improvement, and the reporting of these activities within an organization.
Learn how quality management system components affect inspection readiness – watch our webinar.
The Avoca Advantage
Consulting
Gain Peace of Mind
Over the past 20 years, Avoca has built a proven process to help sponsors and CROs drive efficiency in clinical trial execution. We developed a 12-component construct to facilitate comprehensive, robust gap assessments of existing QMS.

Remove the Guesswork
Using our construct, we help to ensure your QMS is compliant with current regulatory requirements (e.g., ICH E6 (R2) and (R3)) and leading industry practices and standards.

Tools
Fill the Resource Gap
We alleviate time, staff, and other resource constraints that prevent compliance and preparedness assurance — allowing your team to focus on their core responsibilities. To simplify and streamline the successful implementation of your company’s QMS, we leverage the 1,000+ quality management and inspection readiness leading practices, tools, standards, and metrics developed and refined by the Avoca Quality Consortium (AQC).
Leverage Our QMS Consulting Service
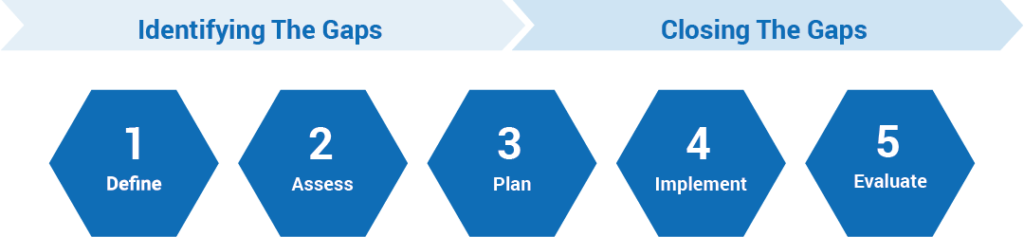
- Collaboratively work with stakeholders to develop the project charter and define the roles and responsibilities.
- Interview key stakeholders to assess the current state and define the desired state.
- Review relevant SOPs or procedures and associated tools and work instructions.
- Identify gaps related to external regulations and leading practice.
- Provide a gap assessment report and implementation roadmap to close the gaps.
- Provide leading practices, tools, and templates to build a fit-for-purpose toolkit.
- Based on the identified gaps and implementation roadmap, Avoca experts assist with:
- Development of:
- Customized training materials.
- Metrics.
- Staffing requirements.
- The strategy and setting the stage for change management, including execution of the communication plan across the organization.
- Revisions to SOPs and/or work instructions.
- Training sessions.
- Conduct of mock audits.