
COVID-19 Consulting Service
|
WCG Avoca’s COVID-19 consulting service provides the assessment, development, and modification support needed for critical decision-making and documentation of processes and approaches in response to the COVID-19 disruption. Our consulting team is comprised of subject matter experts in clinical development and quality assurance and can assess your organization’s current state of COVID-19 preparedness and support you in your recovery and remediation efforts. Ask us about our expert-led clinical trial continuity strategy sessions.
The Avoca Advantage Avoca is a life sciences consulting firm serving the industry for more than 20 years and is home of the Avoca Quality Consortium (AQC), a collaborative of over 120 pharma, biotech, CRO, and clinical service provider companies with the shared objective of elevating clinical trial quality and bringing key stakeholders in the clinical trials process into greater alignment. Avoca COVID-19 Consulting Service Includes:
|
|
Avoca COVID-19 Consulting Process
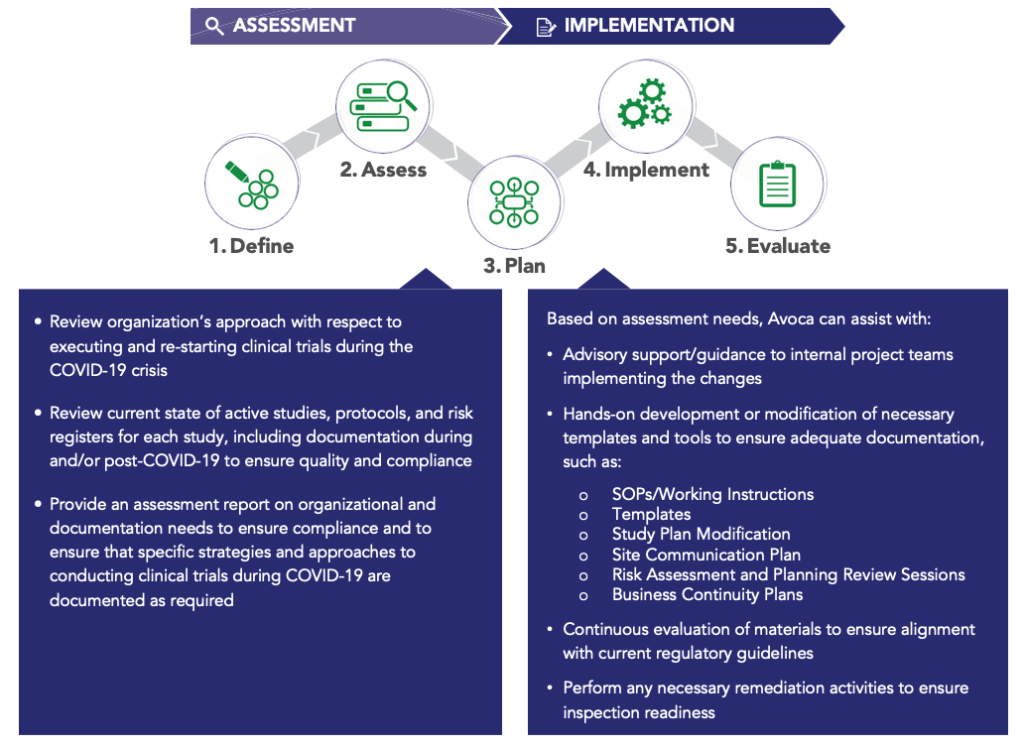
To be contacted to discuss your specific COVID-19 clinical trial needs, please complete the form at the top of this page.
 COVID-19 Rapid Response Working Group
COVID-19 Rapid Response Working Group
Avoca and the AQC have tapped into the existing and successful network of over 120 companies to bring a select group of thought leaders together into a Working Group that meets weekly to share challenges, experiences, questions, and lessons learned as we all navigate COVID-19 and its impact on clinical trial execution.
- The intent is to enable the continuity of clinical trials with strategies and practical support, ensuring that there is a focus on patient safety, data integrity, and regulatory compliance as the COVID-19 situation evolves.
- Avoca has mobilized our panel of clinical trial subject matter experts to support the Rapid Response Working Group and are making these experts available to individual organizations as short-term needs arise.
