Aligning Data Entry and Site Payment Incentives For Clinical Trials and Patients
September 2017
With recent reports indicating that organizations do not have a way of tracking and managing the timeliness of data entry or of site payments, The Metrics Champion Consortium wanted to see if there were opportunities to align incentives in the industry so that both sites and sponsors can achieve their goals with positive impact on clinical trials and patients.
The Metrics Champion Consortium (MCC) recently issued two reports based on industry surveys of data entry timeliness by sites1 and on site payments.2 The reports indicate that, in general, organizations do not have an effective way of tracking and managing the timeliness of data entry or the timeliness of site payments. MCC wanted to see if there were opportunities to align incentives in the industry so that both sites and sponsors can achieve their goals with positive impact on clinical trials and patients.
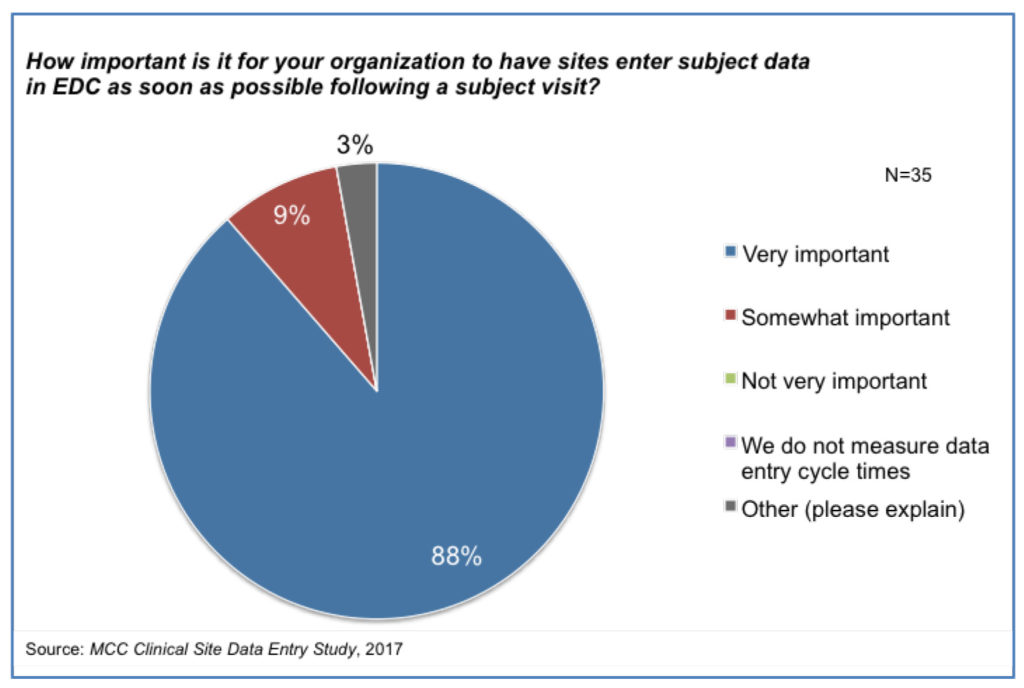
Results from the data entry survey report show organizations consider the timeliness of data entry by sites to be important [Figure 1].1,3 This is not surprising-particularly in light of the implementation of risk-based approaches with Risk Based Monitoring and Quality Management detailed in ICH E6
(R2) Addendum.4 Until data is entered, there can be neither centralized monitoring nor the meaningful remote analysis that is needed for Risk Based Monitoring. With data missing from analyses, it is possible that trends and signals might be detected late and this has a risk for both trial participants and study outcomes. But the site data entry report shows that there is no industry alignment on how to calculate the data entry cycle time metric. The start point is the patient visit. But some organizations define the end point as “one data point” entered, others as “all data points” entered and a few as “all critical data points” entered. Even though the definitions vary significantly, the expectations of organizations for this cycle time do not vary according to the definition. And yet, we would surely expect the cycle time to “one data point” entered to be shorter than the cycle time to “all data points” entered. Most organizations are not aligned with ICH E6 (R2) with their measurement as they do not focus on entry of critical data points specifically. In addition, the report shows that typical performance is far off expectation.
Results from the site payments survey report2 show 80% of organizations either strongly or mostly agreed with the statement, “We consider site payment to be one of our critical processes.” This is not surprising as there have been a number of recent reports on the importance of timeliness of site payments to investigative sites. The Society for Clinical Research Sites (SCRS) found that guaranteed payment in 30 days is considered “very valuable” by 77% of research sites doing more than five studies per year.5 Additionally, SCRS states that 66% of sites report having less than three months’ operating cash on hand, and lengthy delays in payment result in high turnover rates among clinical investigators.5 The Clinical Trials Transformation Initiative (CTTI) cites that as many as 40% of sites drop out of FDA-regulated clinical trials due to lengthy delays in payments.6 No one can doubt the importance of timeliness of site payments to the industry.
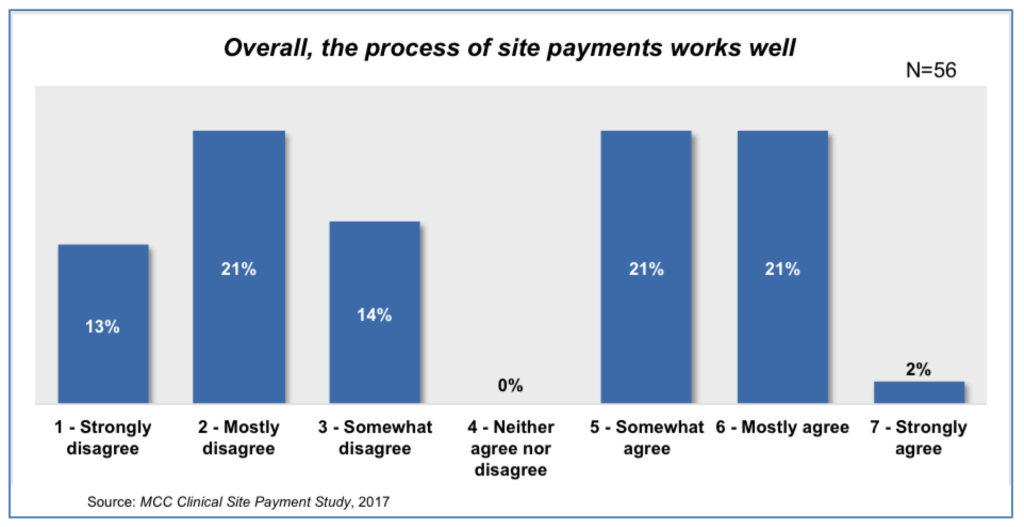
And yet, when asked the level of agreement with the statement, “Overall, the process of site payments works well,” there were very divergent views with nearly half of respondents stating they disagree with this statement [Figure 2].2 CenterWatch has found that fewer than 13% of sites are receiving payment in 45 days or less7 and the MCC site payments report shows that Sponsor expectations of cycle times for site payments are often not met with 56% saying that payment delays occur fairly often or always.2
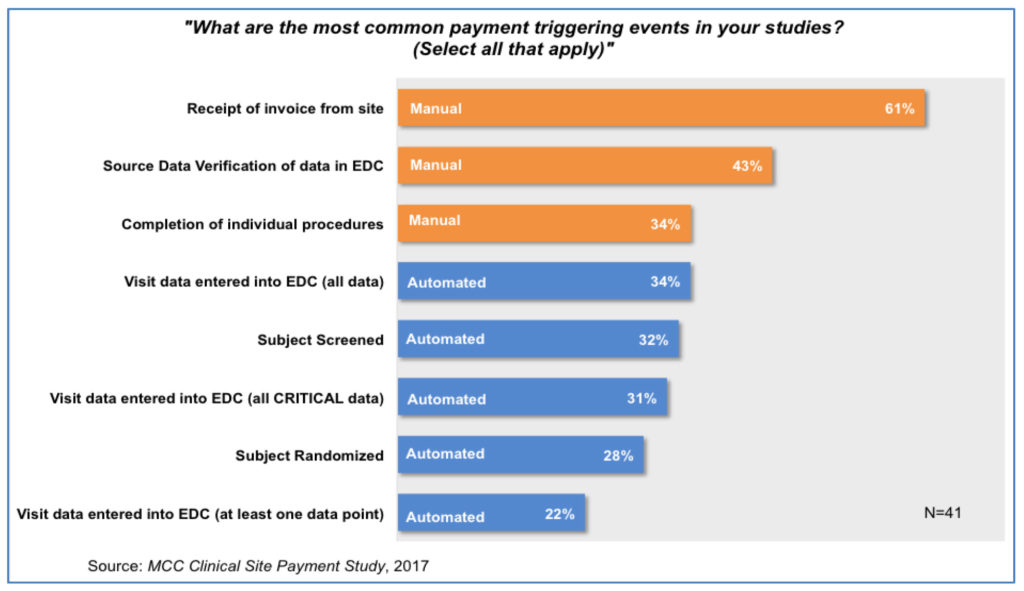
A striking finding is that the most common triggers for site payment are manual ones-namely, receipt of an invoice from site and completion of Source Data Verification of the EDC entries [Figure 3].2 Both of these payment triggers involve significant manual steps to approve and initiate the payment process. The invoice review process is highly prone to error, as the survey demonstrates, and once the invoice is received, the lag time until payment is made to the site is out of the investigative site’s control.2 Similarly, there can be significant time delays awaiting completion of SDV of EDC entries.
This has been suggested by Williams et al.8 although without the specific focus on critical data points. The MCC reports1,2 lend weight to this approach. Sites would have an incentive to enter critical data quickly, which would allow the data to be included in a timely fashion for assessing risk per ICH E6 (R2) Addendum.4 A TransCelerate study9 estimated that only 2.4% of critical data was modified through the SDV process so the vast majority of data entered into EDC is correct. Where invoices are required, these could still be generated automatically and tied to entry of critical data points. This approach would accomplish two things: 1) allow the move away from manual triggers for initiating the site payment process, which are prone to errors and delays, and 2) give the investigative sites more control of the payment cycle time. The approach would also focus effort on critical data as required in ICH E6 (R2) Addendum.4 To implement this would, of course, need consideration of processes, systems, and contracts. There would likely continue to be certain payments that still required a manual process such as invoice generation, but the majority could be more automated.
In addition, this approach would benefit the industry by standardizing definitions of data entry and site payment cycle time metrics and reducing data entry and site payment cycle times. By clarifying and standardizing the definitions, it will be easier to make comparisons and to understand where there are opportunities to improve. Metrics could then be used to demonstrate improvement and to monitor that the improved performance is maintained.
By aligning incentives and connecting the data entry and payment processes in this manner, we can, as an industry, improve our ability to detect risks to trial participants in a timely manner, and help sites manage their cash flow so they can be involved in future clinical trials. Ultimately, this will be to the benefit of patients.
About the Authors
Linda B. Sullivan is Co-Founder and President of the Metrics Champion Consortium. She can be contacted at lsullivan@metricschampion.org.
Keith W. Dorricott is Director of Dorricott Metrics & Process Improvement Limited and a MCC Ambassador. He can be reached at keith@dorricottmpi.com.
References
- Clinical Trial Site Data Entry Study: An examination of how organizations measure site data entry cycle times, performance expectations
compared to actual results and site management strategies. Metrics Champion Consortium Industry Practices Insight Report.
Apr2017. http://metricschampion.org/sitedataentrystudy/ - Clinical Site Payment Study: An examination of how Sponsors and CRO organizations measure site payment processes – trigger events, cycle times, performance expectations compared to actual results and site payment strategies. Metrics Champion Consortium Industry Practices Insight. Sept2017. http://metricschampion.org/ mcc-industry-practice-insight-reports/
- Sullivan LB, Dorricott KW. Importance of Data Entry Timeliness. Applied Clinical Trials. April 2017.
- International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH). ICH HARMONISED
GUIDELINE – INTEGRATED ADDENDUM TO ICH E6(R1): GUIDELINE FOR GOOD CLINICAL PRACTICE E6(R2). Current Step 4 version dated 9 November 2016. Society for Clinical Research Sites. Site Payment White Paper. October 2016 - Fassbender M. Payment tech automates reimbursements to clinical trial sites. Outsourcing-Pharma.com. 20-Jul-2016.
- ACRP – CenterWatch Site Survey 2016
- Williams K, Mackie S. Site Payment Systems And Processes for The 21st Century. Clinical Leader. 14-Apr-2016.
- TransCelerate BioPharma, Inc. Position paper: Risk-based monitoring methodology. May 2013.