Proactive Planning is Key to Process and Quality Improvements in Clinical Trials
Written and submitted by AQC Member, Craig Morgan, Head of Marketing, goBalto™
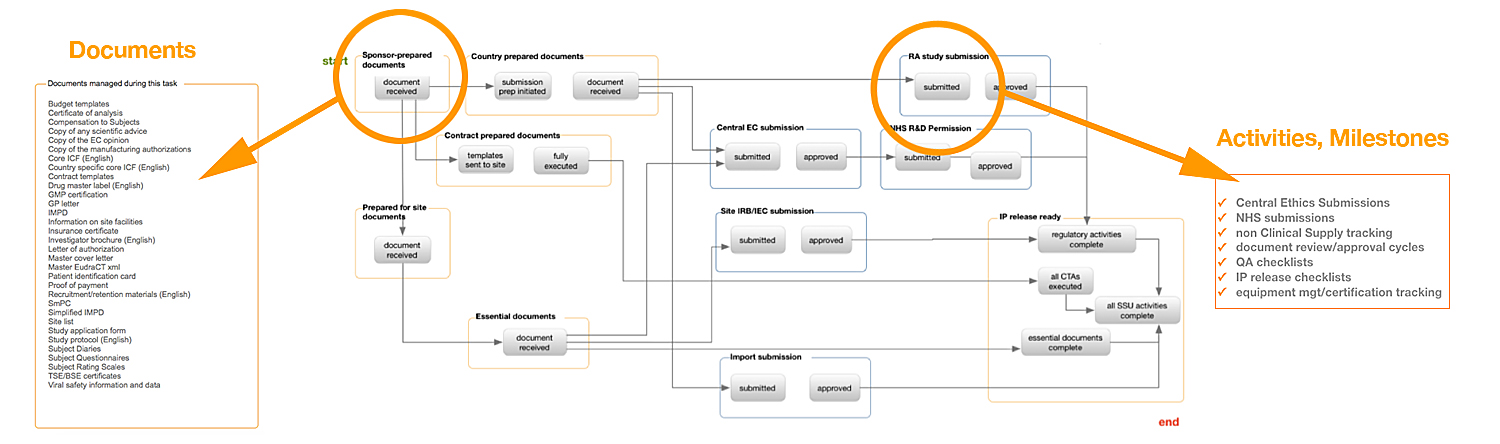
If you fail to plan, you are planning to fail. These words ring true when it comes to study startup, a notorious bottleneck in clinical trials. With research showing a continually stagnating timeframe for conducting clinical trials and a trend toward improving study performance, quality improvement is moving to center stage. With the availability of workflow-based study startup tools (Figure 1), proactive planning, process optimization and quality improvements with study startup—as measured by inspection-readiness and the likelihood of passing regulatory audits—are within reach.
Proactive planning requires sponsors and contract research organizations (CROs) to identify the risks up front, as well as the study requirements, prior to study activation. Failure to do so results in problems not being identified until much later, often in advance of an inspection and well after completed documents, artifacts and metadata have already been released to the trial master file (TMF). A better strategy is to employ processes that take an upfront approach to preventing or mitigating problems associated with study startup document completion.
Fig 1: A country-specific workflow
Using a workflow-based approach to study startup, critical indicators of quality can be assessed on an ongoing basis so that corrective actions can be made earlier (e.g., inaccurate study indexing, missing documents (e.g., missing medical license if you have CV for an investigator), missing document completion date, etc.) This approach can break down silos that have long performed in isolation with little understanding of what the next department needs to fulfill its regulatory obligations and achieve targets measured by performance metrics. Overall, the study improves by greater adherence to timelines, and ultimately by the percentage of artifacts flowing into the TMF that meet quality standards (site activation generates an estimated 40% of all TMF artifacts.)
As the industry turns its attention to better planning, regulatory bodies are drafting regulations to ensure study quality, most notably ICH E6 (R2), which states that the sponsor should implement a system to manage quality throughout all stages of the trial process, including the beginning.
Through workflows, it is possible to launch the planning process by structuring artifacts specific to study activation, while facilitating the exchange of data among eClinical systems. With this capability, any and all needed documents can be defined. Importantly, artifacts and documents can be created 17 weeks before site activation, making it possible to ensure the downstream quality and facilitate inspection readiness at the site level. Making this process change can yield significant improvements to study execution.
Specifically, the regulatory quality assurance process [to ensure inspection readiness] should occur no later than four weeks after site activation, according to the Metrics Champion Consortium quality and methodology definitions*. With the use of upfront workflows, which provide stakeholders with insight months earlier quality can be moved 21-weeks upstream in the process.
Such initiatives to improve quality in early stages of transformational process changes can finally begin to happen as the availability of solutions become available. Research suggests that organizational issues become strategic and of interest to upper management once they have proven relevance to performance.
Learn more about leading practices for proactive study startup planning. View the recording and slides from a webinar, co-presented by The Avoca Group and goBalto on Thursday, May 3, 2018. Topics include how to effectively deliver on improved overall study quality while supporting regulatory compliance for provider oversight. Access the recording and slides here >>
*MCC TMF Implementation Scenario, Page 9.