It’s a Benefit, Not a Risk: Understanding and Countering Misperceptions About Decentralized Trials
Weaving decentralized trial (DCT) elements into clinical trial protocols can enhance quality and improve ROI. Unfortunately, as the results of the 2020 Avoca State of the Industry Survey suggest, many sponsors and CROs fail to recognize that value, and many harbor misconceptions about DCTs.
That was the focus of a recent WCG Avoca webinar conduced with Medable, entitled “Quality Through Innovation: Building Protocols with DCT Elements to Improve Study Success.” To give participants a lay of the land, Cristin MacDonald, PhD, Vice President for Client Delivery at WCG Avoca, began with a few findings from the survey.
Respondents—sponsors and provider companies (mostly CROs)—were asked to identify the top drivers for adoption and effective use of DCTs. The top six responses, in order:
- Increasing study participant retention
- Increasing protocol compliance
- Necessity due to COVID-19-related circumstances
- Increased study participant diversity
- Accelerating timelines for individual clinical trials
- Increasing data completeness and accuracy
Unsurprisingly, the top three relate, to some degree, to the pandemic. “There was the need to be able to continue with these protocols and getting these patients their treatment in a new way, and part of that is also to improve protocol compliance,” she explained. DTC addresses sponsor/CRO concerns about getting patients to stay in a study when they don’t want to visit the site? If they don’t want to come on site, how can the sponsor comply with the protocol?
The next three related to improving clinical trials in general, suggesting respondents see the benefits of DTC extending beyond the pandemic.
MacDonald then shared respondents’ top five perceived challenges to effectively using DTCs:
- Operational/process integration
- Regulatory challenges
- Lack of understanding of the innovation
- Unclear value proposition/uncertain ROI
- Start-up costs
The primary challenges relate to operational and process integration. MacDonald spelled out a few of the concerns: “How do we make this work with a bunch of different sites using different DCT components?” “How do we integrate that into our process?” Regulatory challenges relate to those concerns, while also reflecting the uncertainty over telehealth restrictions and COVID-related FDA guidance.
Lack of understanding—the third greatest challenge—may be the most worrisome. If sponsors and CROs don’t understand decentralization, they will find it hard to grasp its role in the clinical trial process. It will also be difficult to understand the value proposition, which is evidenced by the fourth and fifth items.
Benefit or Risk? Depends on Whom You Ask
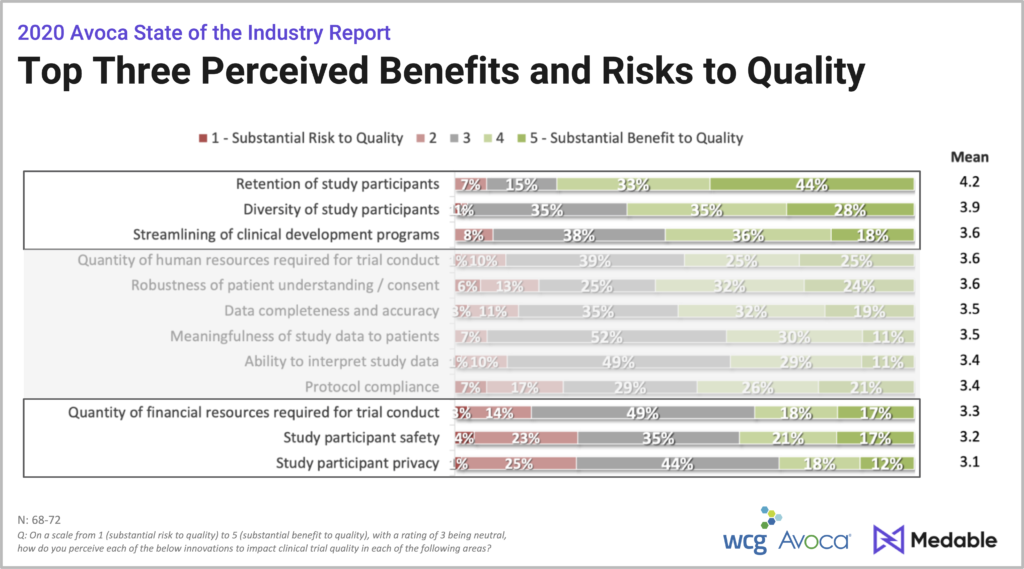
The discussion moved on to another set of responses. What were the top perceived benefits and risk to quality?
The top three perceived benefits:
- Retention of study participation.
- Increased diversity of study participations
- The ability to streamline clinical development
The top three perceived risks:
- Study participant privacy
- Study participant safety
- Financial resources required
What is particularly telling about these findings is that the very aspects that some respondents considered a “substantial risk” to quality were perceived as “substantial benefits” by others, observed Andrew Mackinnon, Decentralized Trials Lead at Medable.
For example:
- 24% of respondents identified protocol compliance it as a risk, but 47% identified it as a benefit.
- 27% identified study participant safety as a risk to quality, while 38% identified it as a benefit.
Such results suggest these perceived risks may, in fact, be misperceptions of risk.
The Need for Change Management
Many of the concerns captured by the survey can be characterized as change management issues, Mackinnon explained.
After all, he points out, many elements of decentralized trials were already in discussion—even in progress—before March 2020. Then COVID-19 forced these changes to be made quickly. “This is about an industry which is maybe not as quick at changing as some other industries, it takes us a little bit of time to get used to an innovation and then to adopt.”
Sponsors must understand that creating a successful decentralized trial—or integrating DTC elements in a conventional one—involves more than merely digitizing an existing protocol. “You can’t take a cookie cutter approach to a DCT and then expect to get better retention, better diversity and quicker timelines,” he said.
He has, however, yet to see a situation in which a decentralized approach cannot be implemented. To illustrate, he offered three case examples.
Case Study One: Age Related Macular Degeneration
This case example focused on streamlining clinical development and enhancing the diversity of study participants. It also helps counter concerns about the cost of DTC trials.
The sponsor needed to screen 11,000 patients with age-related macular degeneration for a rare genetic variant. Taking a traditional approach wasn’t feasible, so Medable worked with them on a decentralized approach. It implemented a fully remote screening using a genetic screening kit and an eConsent approach.
Potential trial participants were consented remotely and shipped a genetic test they could return by mail. Those who passed the screening—and only those individuals— visited the site. Proximity to the participants determined site selection. “We identified the patients, and then we identified the sites,” he explained.
This approach yielded dramatic results, accelerating enrollment, reducing the number of sites and lowering costs:
- 50% decrease in patient enrollment time from an anticipated two years to one year
- 75% reduction in the number of sites required from the 100 to 25
- 40% estimated decrease in the total cost of trial, from $50 million to $30 million
This success, he said, provide an answer a common question: “Is it possible to do decentralized trials with greater levels of technology in an elderly population?” Yes, it is.
Case Study Two: Remote Patient Monitoring
This example helps counter misperceptions related to patient safety, showing how DTC enhances safety while reducing patient burden.
The sponsor of an oncology study needed to detect instances of drug-induced pulmonary fibrosis (PF) in a timelier fashion. In a conventional site-based approach, the site may take an oxygen saturation (SpO2) measure weekly, monthly or even quarterly, making it difficult to detect an adverse event. A decentralized approach allows this happen daily. Daily SpO2 sensor measurements & PF-related symptoms are integrated into the study platform for real-time tracking and assessment.
When a problem is identified, the system automatically sends an additional AE-collection questionnaire to the participant, and the site can determine if the patient needs additional interventions.
Daily patient monitoring not only helps mitigate patient risk, but it does so without adding to the patient burden. The Bluetooth device clips onto a finger and automatically transmits the reading to the application on the patient’s phone and makes that reading immediately available to the site and to the study team.
Case Study Three: Fully Centralized Trial
The first two examples represented hybrid decentralization. This third one looked at a fully decentralized study.
The sponsor wanted to efficiently screen and enroll 1,000 participants in a rare disease trial. A traditional approach would have involved multiple sites and a long enrollment period, which is typical for rare disease trials. The decentralized approach enrolled 75 rare disease patients within three weeks.
The study had a single PI. There was no in-person contact between the PI and the participants. All communication, including consent, took place from a patient’s home. So did sample collection. The participants received all the necessary materials for the requisite blood draws and then were directed to a patient service center.
By ensuring the burden was handled by the technology rather than by the site, this report streamlined the process: It accelerated enrollment, improved retention and allowed for greater diversity.
The Big Picture
The perceived challenges and risks WCG Avoca identified in its survey align with what Mackinnon is hearing from clients. These case studies not only show that the benefits of decentralization far outweigh perceived risks, revealing that risks of decentralization are often misconceptions driven by a lack of knowledge and understanding.
They also demonstrate that current DCT solutions create value for trials many perceive as being a poor fit for decentralization, such as AMD trial involving elderly patients. A critical concern across various study types and patient populations is retention: Bringing the study to patients rather than sending patients to the site dramatically reduces the patient burden, thereby improving retention.
Deploying quality-by-design techniques can ensure technology is woven tightly into the protocol design, he says. This is essential for privacy. All three examples protected patient privacy because those protections are built into the design. “Privacy… is largely addressed through the construct of the platform, the architecture and the security that exists.”
For some studies, taking a fully remote approach will never be ideal, he says. “But there are very few studies that are not a fit for some elements of decentralization to look to reduce the burden to sites and importantly participants in studies. I think that it’s really important to look at the early design elements of a study, consider the problems you’re looking to solve and get the right DCT solution in amongst that to fix that particular problem.”
If you are interested in joining the Avoca Innovation Alliance, focused on operationalizing DCTs within your organization, contact us.
RELATED CONTENT:
Webinar: “Quality Through Innovation: Building DCT Protocols”
View Recording ››